NANOSCALE STRUCTURES OF
ASPHALTENE MOLECULE, ASPHALTENE STERIC-COLLOID AND ASPHALTENE MICELLES &
VESICLES
Nature and Characteristics the Complex Systems
of Asphaltenes and Resins
The investigation of the chemical constitution of
petroleum heavy fractions such as resins and asphaltenes is hindered by their
complex nature. The first known scientific application of asphaltene is by the
French chemist
Niépce. According to experts at the Getty Conservation Institute, the first
surviving photograph by Niepce (1826-7) currently in Austin, Texas has been made
exposing a thin layer of bitumen brushed on pewter plate. When exposed about 8
hours in the camera obscura, the image could be produced by dissolving unexposed
bitumen in oil of lavender.
The classic definition of asphaltenes is based
upon the solution properties of petroleum residuum in various so lvents. The
word asphaltene was coined in France by J.B. Boussingault in
1837. Boussingault described the constituents of some bitumens (asphalts) found
at that time in Eastern France and in Peru. He named the alcohol insoluble,
essence of turpentine soluble solid obtained from the distillation residue "asphaltene",
since it resembled the original asphalt.
Marcusson in1945 classified asphaltenes and
resins as follows : (i) Neutral resins are defined as the insoluble fraction in
alkalies and acids and it is completely miscible with petroleum oils, including
light fractions; (ii) Asphaltenes are defined as inso luble fraction in light
gasolines and petroleum ether. In contrast to resins, the asphaltenes are
precipitated in the presence an excess ether; (iii) Asphaltogenic acid is
defined as the soluble fraction in alkaline solutions and in such solvents as
benze ne.
Recently, asphaltene is defined by chemists as
the part precipitated by addition of a low-boiling paraffin solvent such as
normal-pentane and benzene soluble fraction whether it is derived from
carbonaceous sources such as petroleum, coal, or oil shale. S ince asphaltogenic
compounds are present in petroleum in insignificant quantities, resins and
asphaltenes are most important compounds of petroleum. There is a close
relationship between asphaltenes, resins, and high molecular weight polycyclic
hydrocarbo ns. In nature, asphaltenes are hypothesized to be formed as a result
of oxidation of natural resins. On the contrary, the hydrogenation of asphaltic
compound products containing neutral resins and asphaltene produces heavy
hydrocarbon oils, i.e., neutra l resins and asphaltenes are hydrogenated into
polycyclic aromatic or hydroaromatic hydrocarbons. They differ, however, from
polycyclic aromatic hydrocarbons by presence of oxygen and sulfur in varied
amounts.
On heating above 300-400 oC, asphaltenes are not
melted, but decompose, forming carbon and volatile products. They react with
sulfuric acid forming sulfonic acids, as might be expected on the basis of the
polyaromatic structure of these components. The co lor of dissolved asphaltenes
is deep red at very low concentration in benzene as 0.0003 % makes the solution
distinctly yellowish. The color of crude oils and residues is due to the
combined effect of neutral resins and asphaltenes. The black color of som e
crude oils and residues is related to the presence of asphaltenes which are not
properly peptized.
Structure and Chemistry of Asphaltenes and
Resins
Our knowledge of the asphaltenes is very limited.
Asphaltenes are not crystallized and cannot be separated into individual
components or narrow fractions. Thus, the ultimate analysis is not very
significant, particularly taking into consideration that the neutral resins are
strongly adsorbed by asphaltenes and probably cannot be quantitatively separated
from them. Not much is known of the chemical properties of asphaltenes.
Asphaltenes are lyophilic with respect to aromatics, in which they form highly s
cattered colloidal solutions. Specifically, asphaltenes of low molecular weight
are lyophobic with respect to paraffins like pentanes and petroleum crudes.
There have been considerable efforts by analytic chemists to characterize the
asphaltenes in terms of chemical structure and elemental analysis as well as by
the carbonaceous sources. A number of investigators have attempted to postulate
model structures for asphaltenes, resins, and other heavy fractions based on
physical and chemical methods.
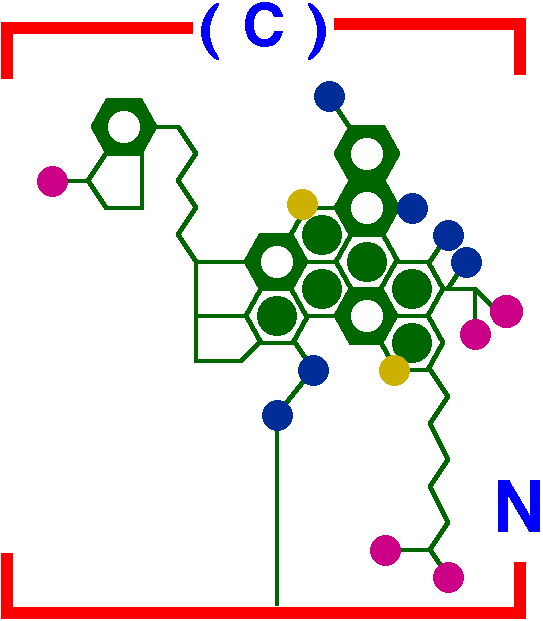
Molecular structure of asphaltene proposed for
Maya crude (Mexico) by Altamirano, et al. [IMP Bulletin, 1986]
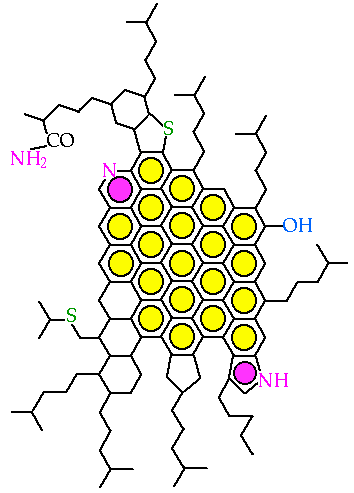
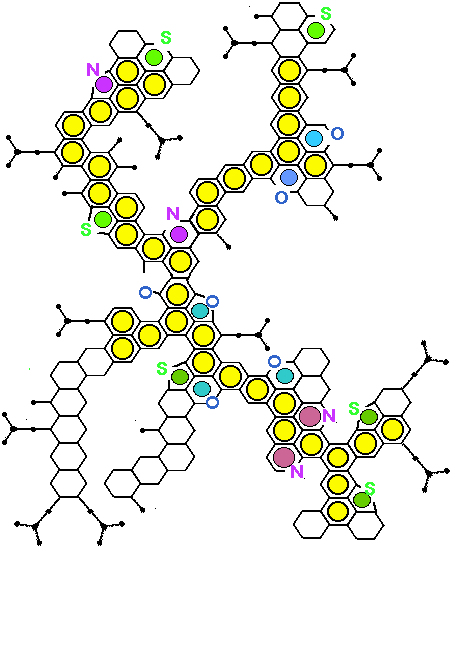
Molecular Structure of Asphaltene Proposed for
510C Residue of Venezuelan Crude by Carbognani [INTEVEP S.A. Tech. Rept., 1992]
Physical methods include IR, NMR, ESR, mass
spectrometry, x-ray, ultracentrifugation, electron microscopy, VPO, GPC, etc.
Chemical methods involve oxidation, hydrogenation, etc.
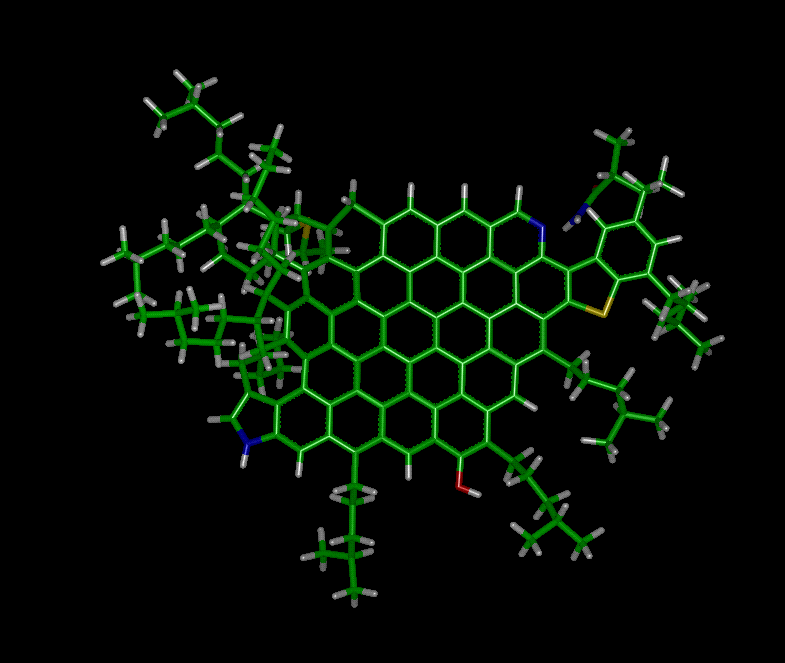
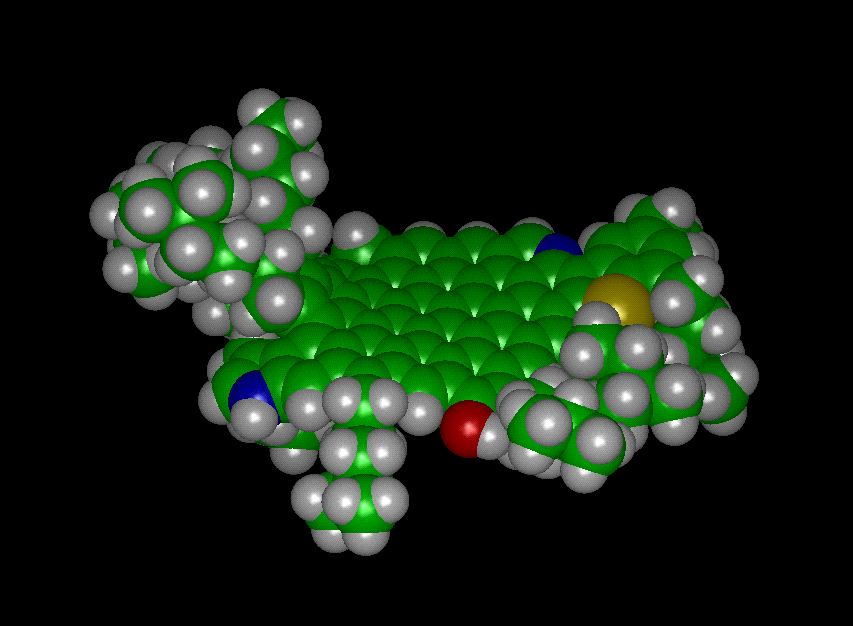
3D Picture of Carbognani's Model of Venezuelan
Crude Asphaltene Molecule (Courtesy of Prof. J. Murgich)
Prof. T.F. Yen in 1974 suggested asphaltene
structure which is deduced from microscopic and macroscopic analysis, showing
their micro- and macro-molecular bonding. From chemical methods, Yen postulated
the micro-structure of asphaltene in which the aroma tic nuclei of petroleum
asphaltene are of a peri-condensed nature and various structural parameters such
as aromaticity, substitution extent, etc. can specify a given asphaltene
structure with hypothetical empirical formula C74H87NS2O. By physical approa
ches such as dissociation-association, charge transfer, excitation, defect
center, etc., Yen also postulated the macrostructure of asphaltene. One of the
properties of peri-condensed polynuclear aromatic systems is the attraction
between the p-electron s ystems. For petroleum asphaltenes, in general, the
stacking is circa five layers as found by x-ray diffraction.
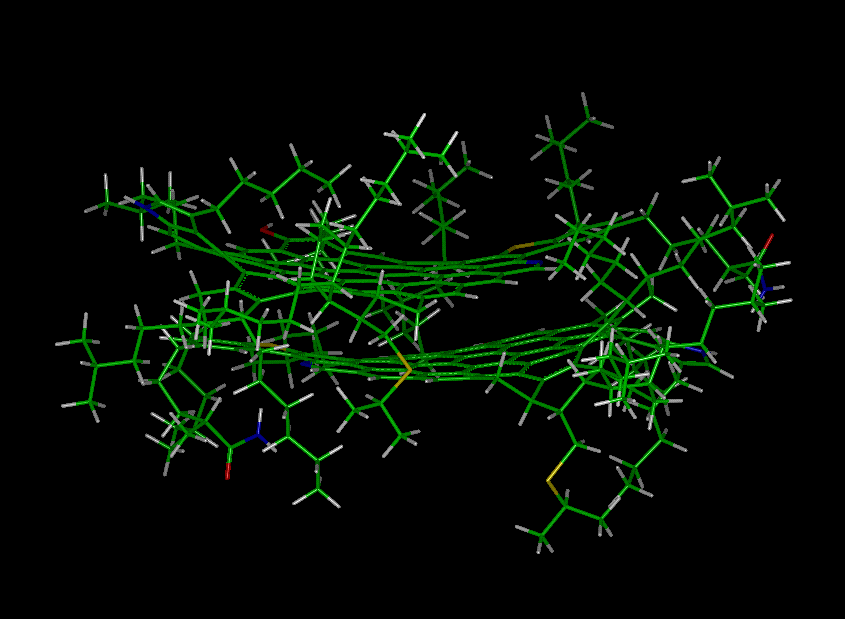
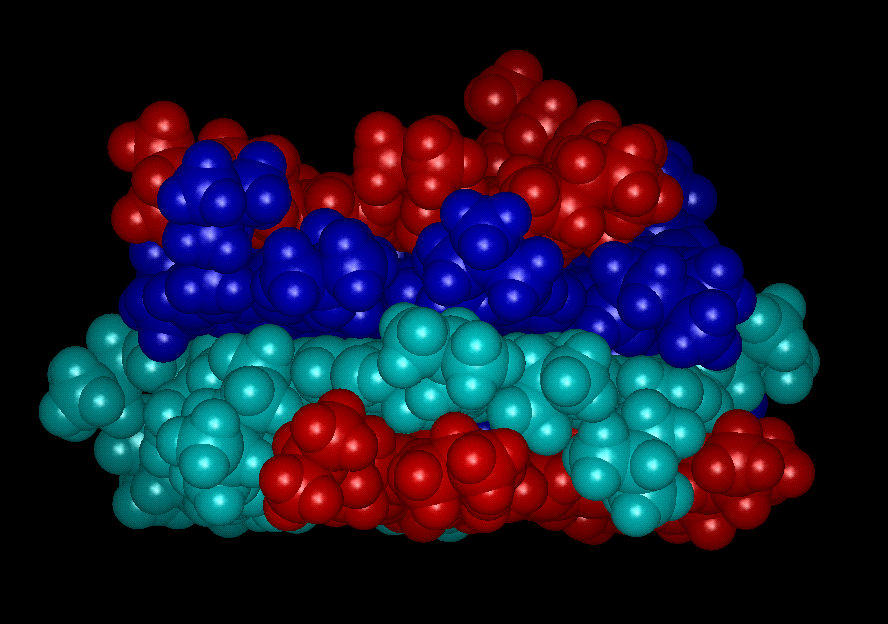
Flocculation/Aggregation of Venezuelan Crude
Asphaltene Molecules (Courtesy of Prof. J. Murgich)
NOTE: Flocs and aggregates of
asphaltene which are caused due to the addition on non-polar solvents must not
be confused with asphaltene micelles which may be formed by the addition of
large amounts of polar (or aromatic) solvents to the crude oil.
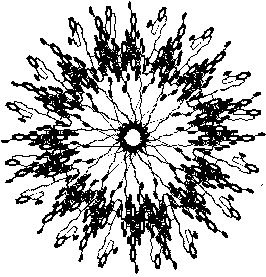
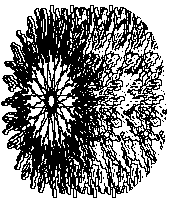

Various shapes of asphaltene micelles formed
in the presence of large amounts of polar or aromatic solvents
Size of Asphaltene Particles
The physical and physico-chemical properties of
asphaltenes are different from those of neutral resins. The molecular weight
of asphaltenes is very high ranging from approximately 1,000 to 2,000,000. The
reported molecular weight of asphaltene varies cons iderably depending upon
the method and conditions of measurement. A major concern in reporting
molecular weights is the association / aggregation of asphaltenes which can
exist at the conditions of the method of measurement. Vapor pressure osmometry
(VP O) has become the prevalent method for determining asphaltene molecular
weights. However, the value of the molecular weight from VPO must be weighed
carefully since, in general, the measured value of the molecular weight is a
function of temperature, the solvent molecular properties. Reported molecular
weights from ultracentrifuge and electron microscope studies are high. To the
contrary, those from solution viscometry and cryoscopic methods are low.
By careful work of electron micrography with
rapid lyophilization, the size of asphaltene is found to be 20-30 by Dickie
and co-workers in 1968. According to Dwiggens (1965) and Pollack and Yen
(1970) in native oil and solutions, the asphaltene partic le size can be
doubled. If the internal structure of a particle is known then, technically,
one can predict its size by taking into account the bond lengths.
The relevance to Heavy Organics Depositions
from Petroleum Fluids
Asphaltenes and resins are two of the several,
but important, heavy organics present in petroleum fluids. Their exact
molecular structures are not generally known in a particular oil field and
they could vary from well to well. In developing the know- how to mitigate and
prevent heavy organics deposition from petroleum fluids exact knowledge of the
molecular structures of all the heavy organics present in a particular oil
field are not necessary. What we need to know is their various macroscopically
d etectable roles in such depositions as it is discussed in a short course
entitled
Experimental Methods in Characterizing Petroleum Fluids and Heavy Organics -
Field and Labora tory Techniques offered by the UIC/TRL.
The mathematical models developed at the
UIC/TRL using polydisperse
statistical thermodynamics, mechanics, kinetics and transport for phase
transitions, interactions, aggregations and depositions, (
ASPHRAC), requires to identify these certain macroscopic measurable
properties which could then quantify the role of the heavy organics in a
petroleum arterial blockage problem.
The importance of the molecular structures of
asphaltenes and resins to the practicing engineers is similar to the
importance of the knowledge of a cardiologist about the structure of
cholesterol present in the arteries of a patient.
Back to
UIC/TRL Heavy Organics
Depositions Home Page
Para mayor información envíe un mail a
info@e-asfalto.com
TELFAX: 005411-4754-9374 /
6351-6288 (whatsapp) / 5931-8727
Buenos Aires - Argentina -
Last update: 02/17/02
HOME